-
Products
- Lab Instruments
- Lab Meters and Probes
- Chemistries, Reagents, and Standards
-
Online Analyzers
Ammonium Analysers Ammonia Monochloramine Chlorine Analyzers
- CL17sc
- CL10sc Amperometric
- 9184 sc Amperometric
- Ultra Low Range CL17sc Colorimetric Chlorine Analyser
EZ Series Analysers- Iron
- Aluminium
- Manganese
- Phosphate
- Chloride
- Cyanide
- Fluoride
- Sulphate
- Sulphide
- Arsenic
- Chromium
- Copper
- Nickel
- Zinc
- Ammonium
- Phenol
- Volatile Fatty Acids
- Alkalinity
- ATP
- Hardness
- Toxicity
- Sample Preconditioning
- Boron
- Colour
- Nitrate
- Nitrite
- Silica
- Hydrogen Peroxide
- EZ Series Reagents
- EZ Series Accessories
- EZ sc Series Inorganics
- EZ sc Series Metals
- EZ sc Series Nutrients
- Flow
-
Online Sensors and Controllers
Digital Controllers (Transmitters) Controllers (Analog)
- SC4500
- CA9300 Series Analog Transmitters
- Orbisphere 410/510 Carbon Dioxide
- Orbisphere 410/510 Oxygen
- Pro Series
pH & ORP Sensors- 12mm pH/ORP
- 8362 sc High Purity
- Combination pH/ORP
- Differential pH
- Digital Differential ORP
- Digital Differential pH
- LCP ORP
- LCP pH
- Sampling
- Multiparameter Online Panels
- Claros Water Intelligence System
- Test Kits & Strips
-
Microbiology
Prepared Media
- BARTS
- Liquid MPN
- MUG Tube
- Membrane Filtration
- Paddle Testers
- Presence-Absence
- Total Count Media
- Yeast and Mold
Dehydrated Media Kits Cryptosporidium & Giardia Analysis Legionella pneumophila Test Pseudomonas aeruginosa TestLabware- Accessories
- Funnels, Pumps & Manifolds
- Microbiology Filters
- Petri Dishes & Accessories
- Sampling Bags
- Vials, Tubes, Bottles & Racks
- Comparators
- Microbiology Accessories
- Microbiology Chemicals
- QC: Microbiology
- Quanti-Trays
- Sealer and Rubber Inserts
- UV Lamps
- Vessels
-
Lab Equipment and Supply
Apparatus
- Brushes
- Clamps, Rings & Stands
- Crucibles
- Crucibles & Casseroles
- Dispensers & Droppers
- Grab Samplers
- Oil and Grease
- Other Apparatus
- Pipet Aids
- Pipettes
- Racks
- Stir Bars
- Tubing
- Weighing Accessories
Instruments- Balances
- Hot Plates & Stirrers
- Microscopes
- Moisture Analysers
- Other Instruments
- Ovens & Incubators
- Thermometers
- Timers
- Vacuum Pump
- Automated Lab Systems
-
Environmental
Accessories Ambient Weather
- Kipp & Zonen Pyrgeometer
- Kipp & Zonen Scintillometer
- Lufft Ultrasonic Wind Sensor
- Lufft WS Series Smart Weather Sensor
- Lufft WS10 Series
Visibility and Present Weather Detection Hydrology Software Hydrology Water Discharge - Flow Road and Runway Sensors Solar Tracking and PV SoilingWater Level- OTT CBS Compact Bubbler Sensor
- OTT PLS Pressure Level Sensor
- OTT PLS(-C) Pressure Level Sensor with Conductivity
- OTT RLS Radar Level Sensor
- SUTRON Accubar Pressure Level Sensor
- SUTRON Constant Flow Bubbler
- SUTRON SDR Stage Discharge Recorder
Data Loggers and Telemetry- ADCON - Wireless Radio Communication / Telemetry
- Kipp & Zonen Data Logger
- SUTRON Antenna
- SUTRON SatLink 3 Logging Transmitter
- SUTRON XLINK 100 Logging Transmitter
- SUTRON XLINK 500 Logging Transmitter
- SUTRON XLite 9210 Datalogger
- SUTRON Xpert2 Datalogger
- Parameters
- Applications
- Industries
- Brands
- Service & Support
- News & Events
Hach Malaysia
Choose your country or region:
Europe
Americas
Asia - Australasia
- Australia
- Mainland China
- India
- Indonesia
- Japan
- Malaysia
- New Zealand
- Philippines
- Singapore
- South Korea
- Thailand (Thai)
- Taiwan
- Vietnam
Middle East - Africa
Chemistries 603-2779 2080
Chloramination
Is Chloramination right for you?
Chloramination disinfects drinking water by adding chlorine and ammonia to achieve a residual of monochloramine. Chloramination is favored for disinfection when there is a need to reduce the risk of disinfection byproducts (DBPs) formation and to extend the useful life of residual in the distribution system.
Understanding of the breakpoint chlorination curve will help you control the process and minimize the formation of undesirable chloramines, like dichloramine and nitrogen trichloride.
Watch the video to understand chloramination and the breakpoint chlorination curve
What is Chloramination?
Chloramination sometimes replaces chlorination to accomplish more reliable disinfection, because it can reduce the likelihood of formation of certain disinfection byproducts (DBPs) and provides a longer-lasting residual in distribution systems. Chlorination typically refers to application of chlorine to achieve a residual of free chlorine. Chloramination refers to purposeful reaction of chlorine with ammonia to create residual concentration of monochloramine, NH2Cl.
Explore Methods and Parameters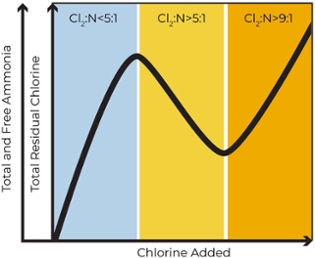
Why Chloramination?
Chloramines have lower reactivity compared with free chlorine, and they react less intensely with various impurities in raw water, particularly organic substances. This results in the formation of fewer carcinogenic DBPs, primarily trihalomethanes (THMs). This is one of the most important factors contributing to the demand for replacing chlorination with chloramination. Also, because of reduced oxidizing power, chloramines create a substantially smaller disinfectant demand, which significantly reduces chlorine consumption to maintain a desired total chlorine residual in the water, which lowers the treatment cost.
Explore Methods and Parameters
Why test with Chloramination?
Proper control of the chloramination process is critical to successful formation and maintenance of monochloramine while minimizing or eliminating the possible formation of undesirable chloramines (dichloramine and nitrogen trichloride) and chance of nitrification.
With analytical testing, you can:
• Optimize monochloramine formation
• Meet total chlorine residual regulatory requirements
• Determine and maintain the best ammonia & chlorine feed pump rates
• Reduce the risk of nitrification events

Additional Resources

Use Process Control as the Basis for Efficient Chloramination
Learn why monitoring monochloramine and free and total ammonia is the most effective way to control chloramination. And understand how online instrumentation gives you more efficient process control compared to laboratory measurements.
LEARN MORE
Nitrification Control with Monochloramine and Free Ammonia Testing
Learn how the Florida Keys Aqueduct Authority eliminated free ammonia in their water to control nitrification.
LEARN MORE
How to Maintain a Continuous Target Ratio of Chlorine and Ammonia
Maintaining the precise ratio of chlorine and ammonia during disinfection is highly challenging. Learning how online instrumentation and integrated process control can improve your target ratio.
LEARN MORE
Chloramination Case Study
Learn how the City of Corpus Christi used the SL1000 PPA to battle nitrification issues.
Fast Field Testing
Learn how to use the Hach SL1000 Portable Parallel Analyzer for fast, accurate, and easy field testing.
Prevent Nitrification
The 5500sc Ammonia Monochloramine Analyzer provides all the information you need to prevent nitrification.
Which Options are Right for You?
Chlorination is a multifaceted process that involves a variety of factors, and every facility and operation is different. Whatever your needs, Hach is ready to help with information, technology, and support.
While chloramination provides some benefits over chlorination regarding the formation of fewer disinfection byproducts (DBPs) – particularly trihalomethanes (THM) that exhibit carcinogenic properties – it requires thorough monitoring to maintain control and ensure optimal results. Successful chloramination is based upon monochloramine formation from free chlorine and ammonium and this process requires strict control to prevent unintended consequences such as nitrification in the water network. In order to achieve the necessary control and better optimization of water treatment processes, operators must carefully monitor formation of monochloramine as the target disinfectant.
Explore the different types of chlorination parameters and methods below.

Primary Parameters
Total Chlorine
Test for total chlorine to determine total disinfectant residual levels in produced potable water. The total chlorine concentration is required by most regulatory agencies for compliance reporting purposes. Use the total chlorine concentration for calculating the CT credits and optimizing the treatment process. Select the DPD Total Chlorine method that adequately covers the expected chlorine concentration and gives the test sensitivity and concentration resolution desired. The method required may change depending upon the sampling point within the treatment system. The titration methods are easily adapted to meet the test ranges required in the treatment process.
Monochloramine
Test for monochloramine to optimize the chloramination process by monitoring the formation of the target disinfectant species and preventing the formation of the less desirable dichloramine form. Test for monochloramine to study reaction rates and mixing rate efficiencies during the formation of the chloramines. The monochloramine test is used together with a free ammonia determination to reduce raw material costs by preventing the over-feeding of chlorine and ammonia.
Free Ammonia
Measure free ammonia (unreacted ammonia) to control and optimize the formation of chloramines. A level below 0.10 mg/L as NH3-N (or NH4-N) is a desirable target level. Excess free ammonia can lead to nitrification problems in the finished distributed waters. Excess free ammonia can react with free chlorine to increase monochloramine and total chlorine residual levels. The absence of free ammonia may indicate that excess free chlorine has been added and that the chloramination process has progressed past the optimum monochloramine formation and the less desirable dichloramine is being formed. The Free Ammonia Indophenol method is designed to measure free ammonia in the presence of monochloramine. Traditional Nessler method have a direct interference from chloramines and should not be used in chloraminated waters.
Find the right testing solution
Secondary Parameters
Total Ammonia
Measured as a sum of all ammonia nitrogen present in the solution in the form of monochloramine (NH2Cl), ammonium ion (NH4+), and molecular ammonia (NH3). This parameter may serve as a primary or secondary verification to maintain the chloramination process under control.
Free Residual Chlorine
Use the No-interference indophenol free chlorine method to study the disappearance of free chlorine in reaction and chloramination process optimization studies without interference from monochloramine. Verify potential excess of free chlorine indicating overchlorination, in addition to measuring free ammonia concentration.
Find the right testing solution
Additional control parameters
pH
Monitor pH to maintain a reasonably steady pH value and to control process efficiency. While pH levels may be unique to a specific chloramination process, a pH range of 8.0 to 9.0 is most effective in the forming and sustaining of monochloramine. Lower pH levels tend to increase the formation of dichloramine, especially in processes where ammonia is added to chlorinated water and poor or slow mixing of the ammonia and chlorine is occurring. The pH value is used in determining CT credits.
Nitrite
Nitrite is formed when ammonia-oxidizing bacteria convert ammonia. The health-based maximum contaminant level (MCL) of nitrite is 1 mg/L for the source water monitoring stage but is not regulated at the distribution stage. If nitrite exceeds the MCL, it can still be a public health concern. It’s critical to watch first for preliminary indicators such as dropping monochloramine and increasing free ammonia. However, decreasing levels of pH and dissolved oxygen with nitrite rising above detection level is a clear indication that nitrification has begun. Trend analysis for these parameters becomes the key to successful monitoring of the distributed water quality and prevention of nitrification.
ATP
Measure total active microorganism levels, which indicate contamination risk, to provide real-time, actionable data and keep your drinking water process running smoothly and efficiently. Rising levels of bacteria can result in consumption of ammonia and oxygen. A combination of biological and chemical (ATP) monitoring and trend analysis is the best approach to ensure the health of your chloraminated systems.
Dissolved Oxygen
Dropping levels of dissolved oxygen and pH is an indicator of nitrification associated with bacterial oxidation of free ammonia. It’s advisable to continuously monitor levels of dissolved oxygen to be able to analyze the trends to ensure nitrification control and the health of your system
Find the right testing solution
Auxiliary/trouble-shooting parameters
To ensure successful chloramination, it is also important to measure raw materials such as hypochlorite and aqua ammonia, which are unstable and may decrease in concentration during transportation and storage. It is also necessary to measure interferences such as iron and manganese to ensure the success of the disinfection processes.
Total Ammonia
Bulk hypochlorite (bleach) solutions are unstable and the chlorine concentration will decrease during transportation or during storage. Delivered solutions are billed based on the % or g/L chlorine concentration delivered to the facility or enduser. The chlorine concentration is checked upon delivery to ensure accurate billing. Storage tanks are periodically checked to maintain optimum chlorine feed pump rates. Test methods designed to measure % or g/L chlorine concentrations directly without dilution should be used.
Aqua Ammonia
Bulk aqueous ammonia (Aqua Ammonia) solutions are unstable and the ammonia concentration may decrease during transportation or during storage. Delivered solutions are billed based on the ammonia concentration delivered to the facility or end-user. The ammonia concentration is checked upon delivery to ensure accurate billing. Storage tank concentrations are checked to maintain the optimum ammonia feed pump rates required to maintain the desired chlorine to ammonia ratio when forming chloramines. Test methods designed to measure % or g/L ammonia concentrations directly without dilution should be used.
Iron
Residual iron is checked to monitor the effectiveness of the chlorine pre-oxidation processes used to remove iron. Use the FerroVer Iron colorimetric method for routine testing. Use the TPTZ Iron colorimetric method when low-level iron testing is required.
Manganese
In addition to causing operational and aesthetic problems, manganese causes a false positive value in standard chlorine DPD analyses. The positive interference cannot be readily quantified with the DPD determination. The presence and concentration of manganese must be separately analyzed to determine if sample pretreatment is required or compensate for the manganese interference. Test also to check the effectiveness of the manganese removal processes. Use the Indophenol method (Monochloramine, Free Ammonia) over the DPD total chlorine, especially if manganese is present.
Find the right testing solution
Select the attributes below to find the right chloramination product for you.
Hach has a range of chloramination solutions that can work in combination to ensure confidence in the quality of your drinking water. While solutions like the 5500sc AMC continuously monitor your chloramination process, the SL1000 PPA can be used to verify your results. In addition, we have lab, online, and portable solutions for various parameters so you can test your water quality anywhere.
Compare our different products used for various applications by exploring the resources and options below.
PRODUCT SUPPORT
CUSTOMER SERVICE
RESOURCES
ABOUT US
Looking for our Hach US Site?
It appears that you are visiting Hach from the United States but the site you are currently visiting is for Malaysia.
Would you like to visit the Hach US site instead?



